Explanation of Nickel and Chromium Layers
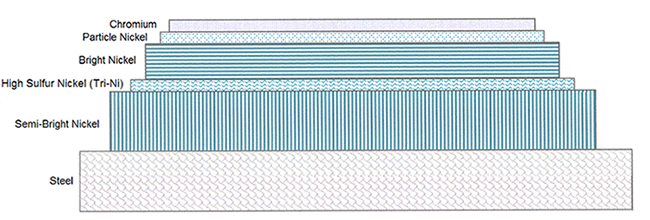
Duplex Nickel refers to the combination of the Semi-Bright Nickel layer which is a sulfur free layer that has a vertical (columnar) grain structure and the Bright Nickel layer which has medium sulfur content and a horizontal (laminar) grain structure. The Semi-Bright Nickel layer with its columnar grain structure provides the bulk of the corrosion protection in outdoor applications. The Bright Nickel layer with its laminar grain structure provides a bright, high luster appearance. The following nickel layers enhance the corrosion protection of the Duplex Nickel system:
- The High Sulfur Nickel layer slows the chemical corrosion reaction by acting as a sacrificial layer due to the increased electrical potential difference between the High Sulfur layer and the sulfur free Semi-Bright Nickel layer.
- The Particle Nickel layer allows the Chromium deposit to become microporous, having 64,000 pores or more per square inch. This high density of pores slows down the corrosion rate by only allowing small chemical corrosion cells to develop in the chrome surface.
- The final layer is the Chromium deposit. The purpose of the Chromium layer is to prevent the top nickel layer from discoloring, to provide for added corrosion protection when it is microporous and to produce a silver-like appearance.
The combination of all of these nickel and chrome layers allows Century Plating to produce finishes that excel in corrosion protection and appearance. Using this process we can meet or exceed all of the outdoor nickel-chrome plating specifications. We also apply this process competitively to indoor applications and thereby provide enhanced appearance along with greater corrosion protection.
